|
|
| |

Import of foods, food additives, equipment and containers/packages
According to Inspection Guidelines for Imported Foods etc.
Article 3 (Processing procedures for pre-arrival
import report)
Commissioners of local Korea Food and Drug Administrations
or Directors of National Quarantine Stations should complete
inspection of the pre-arrival import report that is submitted
five days prior to the expected date arrival of imported
foods, food additives, equipment and containers/packages
(If the report is submitted three to five days prior to
the arrival of imported foods etc.,these days will be
counted as two days as the actual number of days.) and
upon warehousing |
|
 |
of consignments at the bonded area, immediately issue a certificate
of import report for the imported foods etc. whose report was
inspected or immediately conduct sampling for the imported foods
etc. that must undergo laboratory inspection.
Article 8 (Inspection of foods etc.)
When an officially recognized inspection laboratory conducts
a random sampling inspection of foods or conducts a laboratory
inspection or the random sampling inspection for equipment,
packages and/or containers, items to test shall follow the Appendix
3 Primary laboratory analyses list of imported foods etc. However,
the Local Commissioners or Directors can add the test items,
when needed.
The tests of pesticide residue in the imported foods etc.
are conducted according to the provisions based on Appendix
2 Instructions for Pesticide Residue Analysis for each classification
of imported agricultural products in Appendix 1. However, the
inspections, conducted by orders from the commissioner of KFDA,
are excluded.
For questions,contact us by phone,fax or e-mail.
For more information, refer to
Korea
Food & Drug Administration homepage.
Import of Animals and Animal Products
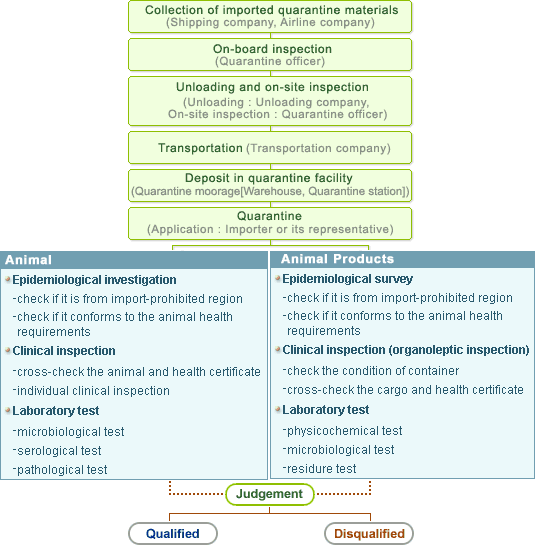
Quarantine inspection for imported animals
1) Submission of Animal Import Plan
Animal Import Plan should be submitted to District office
before the import of animals
in accordance with relevant regulations
2) Report of arrival
Importer of animals should report to District office by
telephone or writing
of the arrival of animals and detailed plan for unloading
and transportation
3) On-board inspection
On-board inspection for vessel is performed in an outer
port On-board inspection for aircraft is performed in a reasonable
area for prevention of livestock epidemic
Inspection items
* Details of transportation
* Health condition during transportation and clinical inspection
* Health certificate issued by exporting country
* Conformity to animal health requirements by Korean authority
4) Unloading and transportation
Unloading and transportation should be carried out in
a safe way to prevent the livestock epidemic by unloading company,
transportation company
5) Moorage in quarantine station
Moorage during quarantine period which includes re-inspection
period
6) Application for quarantine inspection
Required documents
* Application form provided by NVRQS
* Health certificate issued by exporting country
* Vaccination certificate required by relevant regulations or
by animal health requirements agreed with exporting country
* Reference documents can be required by quarantine officer
to verify the authenticity of quarantine application or import
license, etc
7) Epidemiological investigation
Review of quarantine application and attached documents
Review of the results of on-board inspection Any other necessary
epidemiological investigation
8) Clinical inspection and laboratory test
Individual clinical check 1∼2 times a day in accordance with
clinical inspection methods Laboratory test in accordance with
test methods by species
9) Judgement
Qualified : Issuance of quarantine certificate
Disqualified : Return, Incineration or Burial
For questions contact us by phone,fax or e-mail.
For more information, contact
National
Veterinary Research & Quarantine Service
Import of Fishery Products
Inspection Targets
Marine animalplants (cut, heat, matured, dried or salted Fishery
animal and plants simply without using food additive or other
materials)
Inspection Procedure
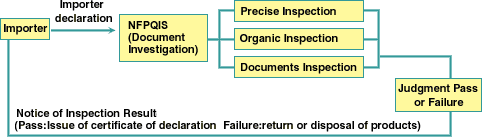
Inspection Methods and Contents
Organic Inspection :appearance, colors, impurities, sorting,
energy,
dryness, flavor of products
Precise Inspection : Inclusion of heavy metal, antibiotic
substance,
paralyzing poison, radioactivity, tar colors, germs, colitis
germs, swellfish poison,
cholera, carbon monoxide of products.
The Flow of Inspection Procedure
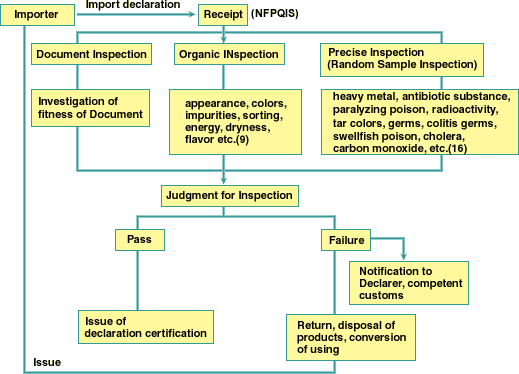
Period for Procedure
Documents Inspection : 2days
Organic Inspection : 3 days
Random Sample Inspection : 5 days
Close Inspection : 10 days
For questions contact us by phone,fax or e-mail.
For more information, contact
National
Fisheries Products Quality Inspection Service
|
|
|
 |
|
|